Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Study of Crystallography and Reciprocal Space of Higher Oxides of Lanthanides Using Electron and Neutron Diffraction
Authors: M. K. Maurya, Harsh Verma, Sunil Kumar Gupta, Shyam Sunder Tiwari
DOI Link: https://doi.org/10.22214/ijraset.2024.63877
Certificate: View Certificate
Abstract
This study explains uses electron and neutron diffraction techniques to study the crystallography and reciprocal space of higher oxides of lanthanides. The significance of crystallography in comprehending the geometric configuration and bonding of atoms in solids is discussed at the outset of the study. The comprehensive analysis of the crystal structures using accelerated electrons and neutrons highlights the tremendous penetration power of these particles. While neutron diffraction is concerned with plane incidence waves and their scattered counterparts, electron diffraction is concerned with electron generation pulsed by a laser.Important findings show how experimental data may be used to validate Bragg\'s equation and show how scattering angles, interplanar distance, and order of wavelength are related. To display the relationship between electron energy and electron radiation per electron volt, energy distribution curves are displayed. Additionally, several crystal systems, such as hexagonal, monoclinic, and cubic face-centered structures, are revealed by structural investigation of higher oxides of lanthanides, such as terbium dioxide (Tb2O3).The talk emphasizes how, in contrast to other elements in the series, lanthanides have special qualities that enable them to produce higher oxides with oxidation states greater than +3. The outcomes highlights the improved precision and in-depth structural insights that come from combining electron and neutron diffraction methods. This opens the door for more studies in the crystallography of complicated materials in the future.
Introduction
I. INTRODUCTION
The field of science is developing on a daily basis. Many of the decades-old riddles are now being answered, and some of the answers have even been improved. The study of crystals is crucial to understanding the vast array of solids, and its importance can propel humanity to new heights.Thus, study of crystallography [1] deals with simplifying the pattern of arrangement & bonding of atoms in a solid. The geometric study of crystal will make the life easier only. It is necessary to acquire knowledge and comprehend certain aspects prior to drawing any conclusions. The most significant question that arises is how a crystal [2] can be defined, the answer is pretty simple; “A material which defines the composition of atoms, whose arrangements are definite & can be characterised just by stamping the surface regularity is known as crystals”.
Since, it is now clear what is a crystal let us define few more scientific terms attached to solids. The smallest unit required to form a very complex structure is called a unit cell. They are generally of two types [3-4]&consists of lattice points among themselves. The three-dimensional arrangement of unit cells in all possible direction gives us a crystal lattice [5]. These structures follow the law of symmetry which has been explained within these literatures [6-7]. The group of crystal lattices are distinguished on the basis of axial length of unit cell & the inter-axial angles; which defines them as crystal system [8]. These are all part of real space [9], which also includes the 14 Bravais lattices [10].
The crystals can be bombarded with various incident lights/waves just to see the regularity & check the arrangement of lattice points. The variation in the intensity of the lights/waves due to inelastic scattering can be observed & thus can be grouped in the same basis as of diffraction [11]. The examples of the diffractions [12-14] has been studied & shown in various articles regarding the phenomenon of modification of light.
In this article, the techniques that has been used to study the structure of crystals with the help of diffraction commends the illumination of the specimen with the laser light. These laser lights are properly given a source of some sort to exemplify the answers of determining the crystal shapes and sizes.
The techniques [15-16] include electron & neutron diffraction out of the all the possible variable forms of the system. When certain X-rays are used over a lattice cell, the information carrying waves are very small & hence the study can not be completely perfect, counter to the other two methods which provide a better scope of information in the same working space produces more opportunity of them to be used. The study of real space [9] only provides a certain data to be analysed but the introduction of reciprocal space or lattice [17] helps in signifying the higher order of resolution, thus more questions can be answered.
Lanthanide elements have been chosen because they can easily form oxides of their own. When these elements which are also known as rare Earth elements [18-19] come in contact with oxygen tend to form oxides. But higher oxides can be seen only in few elements which fulfil the requirement of an oxidation state more than +3, other elements only form oxides of lower transitional states. Various lanthanides can also be seen forming higher oxides in future when they are discovered.
II. METHODOLOGY APPLIED
There are two different diffraction techniques which have been studied, they are: -
A. Electron Diffraction
The steps of finding the structure of a material [20-21] with electron diffraction can be explained as: -
- The primary importance to be noted before finding the structure of material is the size of the crystallites, which should be in the order of 10-6-10-5 mfor convenience.
- The secondary requirement is that of a secondary definite structure of the specimen. For the observer, it is essential to obtain the texture or pattern of the secondary structure over a polycrystalline film or single crystal films can be used.
- During structural investigations, transmission recording is almost always used. It is also used in cases of recording the information through reflection from the surfaces of the specimen.
- The next important determination is that of symmetry & the unit cell of the crystal in structural analysis. This data is carried on the basis of pure geometrical theory.
- The next step includes determination of the co-ordinates of the atoms within the unit cell from the measured intensities. The position of the atoms in the crystal can also be determined by trial-and-error methods.
- The final stage of the investigation is the determination of the degree of accuracy of the results obtained.
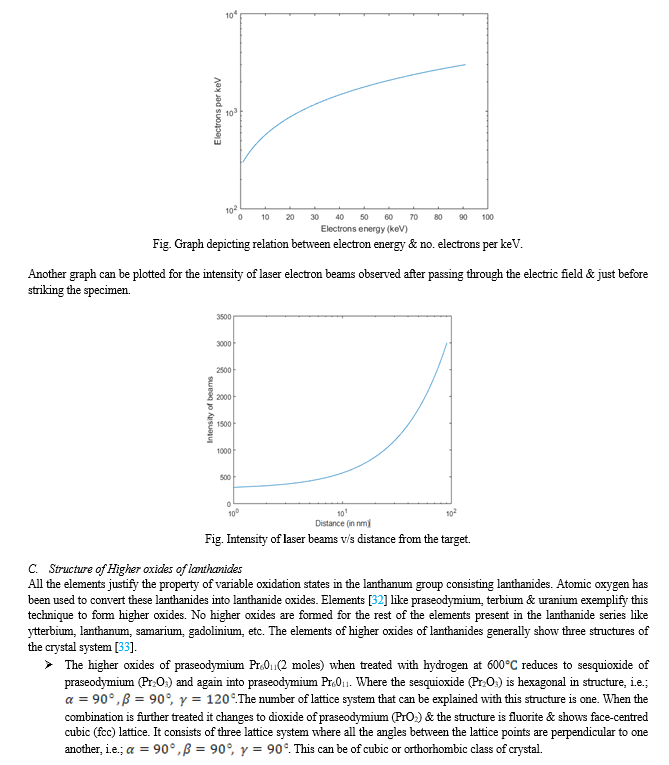
The process used here is a culmination of two different methods.The method applied to show diffraction pattern in the crystal structure is a mixture of 3D electron diffraction using laser plasma electrons. The first part of the method [22-24] used in this technique is used to generate high speed electrons.In these methods, electrons are first generated from a moving steel tape by providing energy to the metal tape. Since, electrons are revolving around the outermost shell of a metal, it makes the process easy to evacuate electron from the orbit of the atoms to its surface. When the electron reaches its potential of leaving the atom, the phrase commonly known as work function [25] is applied. From there, the electron moves in a straight-line path as the moving steel tape is kept in a vacuum chamber so that the excreted electron andother sub-atomic particle do not move in any direction in the environment which may be dangerous even at a laboratory stage. The techniques of ultra-fast electrons have taken the world by surprise. The accuracy shown by this process is more than that of X-ray diffraction; also, various unknown factors which were not being solved by the earlier diffraction techniquesare now being solved by this process under the special arrangement & testimony provided by the regular setup of the experiment.
The experimental setup [22-24]consists of a commercial titanium-sapphire laser emitting 1.6 mJ in 45 fs at a 1 KHz. The repetition rate is focused with a 12 cm focal length lens on a steel tape. The tape is slowly moving in vertical direction and the electrons are emitted horizontally, along the direction of polarization. For collimating, the electrons & to reduce the amount of scattered light, two pinholes of 1 mm & 150 µm in diameter are placed in the beam bath.The electrons thus produced are then incident over the specimen & the 3D images are observed. The laser beam when passes through the two charge plates mutually perpendicular to each other in a landscape pattern attracts or slightly bends towards the opposite charge, which causes the speed of electron to increase. The strength [25] that allows us to accelerated electrons may include;
a. Firstly, the possibility to have parallel electron probes with a size of a few nanometres which allows collecting diffraction data from sample volumes 2 or 3 orders of magnitude smaller than the ones suitable forms X-ray beams.
b. Secondly, the ability to deliver both diffraction and imaging from the same nano volume allows the combination of reciprocal and direct space information and the experimental determination of crystallographic phases.
c. Thirdly, the strong Coulomb interaction between electrons and matter allows a good signal-to-noise ratio even from very thin samples and an easier identification of light atoms, like lithium and hydrogen, when compared with X-rays.
d. The most important one of all the strength & feature is the ability of strong scattering by electrons which indulges us more towards its use.
The second part of the setup includes the detector, analyser and the specimen being incident by the laser beams of electrons. 3D ED technique [26] has been studied as a conceptually comparable technique to a single crystal X-ray but this (3D ED) allows collection of data over a much smaller range of volumes.3D ED requires relatively small crystals and can be applied to crystallization products that are considered failures in the eyes of X-ray crystallographers. 3D ED requires relatively small crystals and can be applied to crystallization products that are considered failures in the eyes of X-ray crystallographers. In this process, the collimated electron pulses which are of very high speed, intensity & wavelength. These features only help us in studying the structure of material by strong scattering technique. When these pulses (electrons) incident over the specimen (Terbium dioxide; Tb2O3) multiple scattering events take place within the crystal. The beam of electrons that come out of the system is thus observed by placing a photographic film at all the possible angles surrounding the specimen (Tb2O3) as shown in figure. The electron beams imaged over the photographic film are studied, data are collected & results are drawn on this basis. The next step is collecting data & the process involved in collecting them.
There are basically two ways of data collection [27-28]:-
a. Stepwise Data Collection Method
- ADT (Automated diffraction tomography).
- RED (Rotation electron diffraction).
- PEDT (Precession-assisted electron diffraction tomography).
b. Continuous Data Collection Method
- MicroED (Microcrystal electron diffraction).
- IEDT (Integrated electron diffraction tomography).
- cRED (Continuous rotation electron diffraction).
The data collection has been done with the help of continuous rotation electron diffraction method or abbreviately said as cRED. In this method, the axis of rotation or the goniometer axis keeps on rotating till the complete 3D structure at every possible angle is being taken (the angular difference is adjusted before the start of the process). The observed sample is then studied with the help of rules already been set to distinguish and classify the crystals. The continuous data collection [27] relies on the high stability of the goniometer, since crystal re-centering is impossible, and on the speed of the detector, which should be fast enough to avoid loss of reciprocal space sampling during readout time. The process is at last completed with the dynamical refinement of the scattered waves.
B. Neutron Diffraction
Neutron diffraction or elastic neutronscattering [29] is the application of neutron scattering to the determination of the atomic and magnetic structure of a material. A sample to be examined is placed in a beam of thermal, hot or cold neutrons to obtain a diffraction pattern that provides information of the structure of the material. The technique is similar to X-ray diffraction but due to the different scattering properties of neutrons versus X-rays, complementary information can be obtained. Particularly, neutron diffraction is more helpful for the localisation of light atoms and the determination of magnetic ordering. In easier terms it can be understood that neutron diffraction provides more than X-ray diffraction.
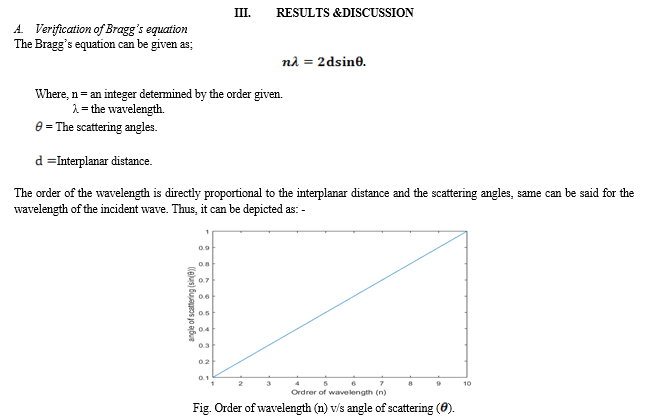
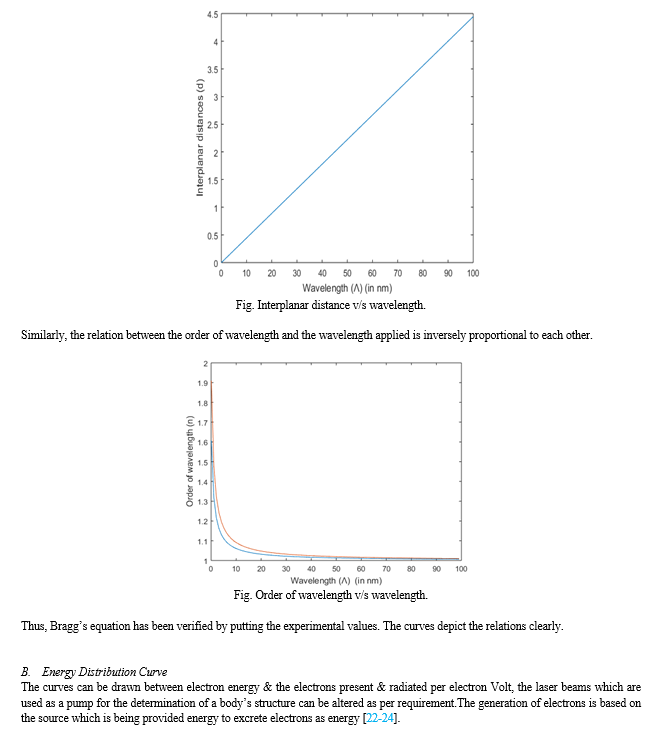
Neutron diffraction is a technique which can be used in studying not just structure of crystals but also the atomic structure of liquids, amorphous materials and crystalline materials. The basic principle of neutron diffraction deals with the study of incident plane wave on a certain specimen and causing both elastic as well as inelastic scattering of waves, which causes changes in the magnitude of wavelength, frequency, & the intensity of a beam of light. This helps in the measurement of the Bragg’s equation (principle).Thus, explanation of Bragg’s equation & its diffraction is also important for neuron diffraction.
The experimental setup [30]clearly depicts the process.The experimental setup consists of a source or a moderator that emits waves with high speed. The next station for the incident waves is the incident beam properties selector which only allows those waves to pass through which has a sufficient a significant wavelength, that is required for that particular observation. The waves then interact with the sample or the specimen which is higher oxides of lanthanide (Terbium dioxide) in this case.
Since, neutrons have a tendency to show elastic scattering thus the part of the energy is transferred for continuous scattering within the crystal & the other part of the energy is passes through the specimen unharmed. The first part of the energy that is being used in scattering; scatters & comes out making an angle from the crystal. This scattered wave is then analysed by the scattering beam properties selector or the analyser. During this process, the energy, wavelength, intensity of the scattered wave is analysed & which is further passed on to the latter stages of conclusion. The last stage of the setup includes detector which detects the shape, size, structure, intensity, wavelength, patterns, etc. of the diffracted wave.
This is further connected to data acquisition system which understands the variation within the system in which the waves were incident. A conclusion can be made by above experiment is that neutron enters as an incident wave & neutrons comes out as a scattered wave too.The neutrons are used as an accelerated sub-particle system to generate a great deal of flux. The energy consumed in this process is very less as the neutrons being accelerated are then incident on the specimen to easily compute the diffraction process & results were used.
The process in itself can be segregated into two: -
- Neutron scattering.
- Magnetic scattering.
The process of neutron scattering can take place from each & every atomic nucleus. The magnetic moment of the atom also provides support in the process of neutron scattering. The incident waves when are made to fall over the specimen, there is a certain system of scattering which can be due to the elastic or non-elastic scattering behaviour. The scattered wave from these experiments is observed and further analysed.
Similarly, magnetic scattering occurs due to themagnetic moment of neutron which can interact with either the orbital or the spin magnetic moment of the material examined [31]. Only because of the greater effect of electron in the magnetic scattering the strength of forward scattering is more than the backward scattering.

Conclusion
1) Bragg’s law and their reciprocal space construction are well confirmed by both Electron ad Neutron diffraction. 2) The higher lanthanides might form oxides by keeping a sheet of rare Earth elements element in air and that is oxygen, the high melting point oxides are formed as thin layer over this metal &later on toLowerCaseJointly. The lanthanides are in the +2 to +4 oxidation state. These ones exhibit variable oxidation number as a result of the fact that either they are half-filled or full. 3) Electron & Neutron diffraction are a better technique than the X-ray diffraction method, because they provide a better information about the shape, size, geometry, lattice structure & presence of lattice points with the density of the material. They directly come in contact either with the nuclei of the material or the electron present in them. Since, neutron diffraction closely resembles the X-ray diffraction it can be used to verify the Bragg’s law & provide a better information of the material, the collection of data has also improved over the time. 4) The values that are already included in the study of actual space or the structural array of any unit dimension, such as length, mass, etc. are what make up the computation of reciprocal space. The method for calculating a value in a real space is the same as that in a reciprocal space; the only thing that differs is that the power of the unit must be changed because a reciprocal space can only compute inverse values. 5) To put it simply, the reciprocal space is produced when the Miller Indices are expressed in inverse value.All lanthanides do not form compounds of higher oxide; only few of them do, those having oxidation state more than +3. Few examples of them are dioxides, sesquioxides of Terbium, Praseodymium, Cerium, etc. & various higher oxides of these elements. The three crystal systems that are present in these compounds are; Cubic “face centered”, Hexagonal, Type “B” (monoclinic or trigonal).
References
[1] Behrens, H., &Luksch, P. (2006). A bibliometric study in crystallography.ActaCrystallographicaSection B: Structural Science, 62(5), 993-1001. https://doi.org/10.1107/S0108768106030278 [2] Goodby, J. W. (1984). Liquid crystal phases exhibited by some monosaccharides.Molecular Crystals and Liquid Crystals, 110(1-4), 205-219. https://doi.org/10.1080/00268948408074506 [3] JieNiu, Choo, H. L., Sun, W., &Mok, S. H. (2018). Title of the article.International Journal of Mechanics and Materials in Design, 14(3), 443–460. [4] Moreno, J., &Soler, J. M. (1992). Optimal meshes for integrals in real- and reciprocal-spaceunitcells.PhysicalReviewB,45(24),13891.https://doi.org/10.1103/PhysRevB.45.1389 [5] IMLifshitz, AM Kosevich - Reports on Progress in Physics, 1966 [6] Houston, W. V. (1940). Acceleration of electrons in a crystal lattice.Physical Review, 57, 184. https://doi.org/10.1103/PhysRev.57.184 [7] Senechal,M.(1986). Symmetry, geometry and crystal symmetry.Elsevier. https://doi.org/10.1016/B978-0-08-033986-3.50042-2 [8] Stokes, H. T., & Hatch, D. M. (2005). Journal of Applied Crystallography, 38(2), 237-238. https://doi.org/10.1107/S0021889804031528 [9] Jullien, R., Fields, J. N., &Doniach, S. (1977). Zero-temperature real-space renormalization-group method for a Kondo-lattice model Hamiltonian. Physical Review B, 16(10), 4889-4896. https://doi.org/10.1103/PhysRevB.16.4889 [10] Zuo, J. M., Weiss, J. K., & Smith, D. J. (1993). Accurate measurements of mean inner potential of crystal wedges using digital electron holograms. Ultramicroscopy, 48(1-2), 1-11. https://doi.org/10.1016/0304-3991(93)90197-6 [11] Cowley, J. M. (1995). Diffraction physics.Elsevier. [12] Warren, B. E. (1990). X-ray diffraction. Dover Publications Inc. [13] Bish, D. L., & Post, J. E. (2018). XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Sustainable Environmental Science, 3(1), 16-29. https://doi.org/10.1016/j.sesci.2017.12.002 [14] Waseda, Y., Matsubara, E., &Shinoda, K. (2011). Scattering and diffraction.X-ray diffraction crystallography (pp. 67-106).Springer. [15] Mills, M. J., Collins, M. D., &Lingevitch, J. F. (2000). Two-way parabolic equation techniques for diffraction and scattering problems. Wave Motion, 31(2), 173-180. https://doi.org/10.1016/S0165-2125(99)00045-1 [16] Whittig, L. D., &Allardice, W. R. (1986). X-ray diffraction techniques. In A. Klute (Ed.), Methods of soil analysis: Part 1. Physical and mineralogical methods (2nd ed., Chapter 12).Soil Science Society of America. https://doi.org/10.2136/sssabookser5.1.2ed.c12 [17] Michael, J. R., &Eades, J. A. (2000). Use of reciprocal lattice layer spacing in electron backscatter diffraction pattern analysis.Ultramicroscopy, 81(1), 67-81. https://doi.org/10.1016/S0304-3991(99)00119- [18] M Bottrill, L Kwok, NJ Long - Chemical Society Reviews, 2006 [19] JL Sabot, P Maestro –Lanthanides. Kirk?Othmer Encyclopedia of Chemical, 2000. https://doi.org/10.1002/0471238961.1201142019010215.a01.pub2 [20] Terwilliger, T. C. (2003). SOLVE and RESOLVE: Automated structure solution and density modification. Methods in Enzymology, 374, 22-37. https://doi.org/10.1016/S0076-6879(03)74002-6 [21] Mathews, D. H., & Turner, D. H. (2002). Dynalign: An algorithm for finding the secondary structure common to two RNA sequences. Journal of Molecular Biology, 317(2), 191-203. https://doi.org/10.1006/jmbi.2001.5351 [22] T Gruene, E Mugnaioli -3D Electron Diffraction for Chemical Analysis: Instrumentation Developments and Innovative Applications,Chemical Reviews, 2021.https://doi.org/10.1021/acs.chemrev.1c00207 [23] M Gemmi, AE Lanza,3D electron diffraction techniques (2019)Acta Cryst.. B75, 495-504.https://doi.org/10.1107/S2052520619007510 [24] Gemmi, M., Mugnaioli, E., Gorelik, T. E., Kolb, U., Palatinus, L., Boullay, P., Hovmöller, S., & Abrahams, J. P. (2019). 3D electron diffraction: The nanocrystallography revolution. ACS Central Science, 5(8), 1315-1329. https://doi.org/10.1021/acscentsci.9b00394 [25] Fill, E. E., Trushin, S., Tommasini, R., & Bruch, R. (2005, September 7). Electron diffraction experiments using laser plasma electrons. Electron Diffraction Experiments using Laser Plasma Electrons. https://doi.org/10.1063/1.2195222 [26] Sung, S. H., Schnitzer, N., Brown, L., Park, J., &Hovden, R. (2019). Stacking, strain, and twist in 2D materials quantified by 3D electron diffraction. Physical Review Materials, 3(6), 064003. https://doi.org/10.1103/PhysRevMaterials.3.064003 [27] Cowley, J. M. (1992). Electron diffraction techniques (Vols. 1 & 2). Oxford University Press [28] Dorset, D. L. (1995). Structural electron crystallography.Plenum Press. [29] Shull, C. G., & Wilkinson, M. K. (1953). Neutron diffraction studies of various transition elements. Reviews of Modern Physics, 25(1), 100. https://doi.org/10.1103/RevModPhys.25.100 [30] Worsham, J. E., Levy, H. A., & Peterson, S. W. (1957). The positions of hydrogen atoms in urea by neutron diffraction. ActaCrystallographica, 10(4), 319-323. https://doi.org/10.1107/S0365110X57000924 [31] Anand, V. K., Adroja, D. T., Hillier, A. D., Taylor, J., & André, G. (2011). Signatures of spin-glass behavior in the induced magnetic moment system PrRuSi3.Physical Review B, 84(6), 064440. https://doi.org/10.1103/PhysRevB.84.064440 [32] Gruen, D. M., Koehler, W. C., & Katz, J. J. (1951). Higher oxides of the lanthanide elements.Terbium dioxide.Journal of the American Chemical Society, 73(4), [page numbers]. https://doi.org/10.1021/ja01148a020 [33] Alvero, R., Odriozola, J. A., Trillo, J. M., & Bernal, S. (1984). Lanthanide oxides: Preparation and ageing. Journal of Chemical Society, Dalton Transactions,(1), 87–93. https://doi.org/10.1039/DT9840000087
Copyright
Copyright © 2024 M. K. Maurya, Harsh Verma, Sunil Kumar Gupta, Shyam Sunder Tiwari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63877
Publish Date : 2024-08-04
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

